Research in the Mansell Group focuses on five interlocking areas:
1. Sustainable Chemistry
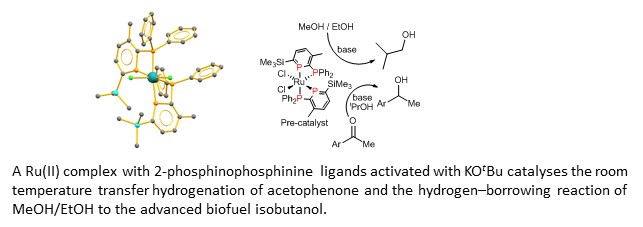
The way we currently make chemicals cannot continue for much longer. The need to use sustainable starting materials and reduce the amount of CO2 released to the atmosphere is driving a change towards sustainable chemistry. We are taking two approaches to this end: Use of phosphinine-metal complexes to explore new reaction pathways, and exploration of known catalysts applied to new synthetic routes that are more sustainable.
Collaborators: Dr John Andresen (HWU), Dr Richard Wingad (Bristol), Lubrizol
Publications
R. J. Newland, M. F. Wyatt, R. L. Wingad and S. M. Mansell, Dalton Trans., 2017, 46, 6172.
R. J. Newland, M. P. Delve, R. L. Wingad and S. M. Mansell, New J. Chem., 2018, 42, 19625.
P. A. Cleaves and S. M. Mansell, Organometallics, 2019, 38, 1595.
E. C. Trodden, M. Delve, C. Luz, R. J. Newland, J. M. Andresen and S. M. Mansell, Dalton Trans., 2021, 50, 13407–13411
2. Small bite-angle ligands: an expanding concept in catalysis
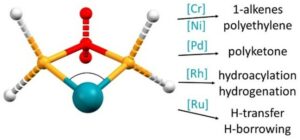
Recent research has demonstrated the importance of the growing area of small bite-angle catalysis, with distinctly different applications to the conventional field of large bite-angle ligands. Based upon the lack of directionality of the phosphinine lone pair, due to its high s-character, we have explored how phosphinines can be utilised to construct catalysts with small bite-angles.
Collaborators: Sasol UK, Dr Natalie Fey (Bristol), Dr Jason Lynam (York)
Publications
S. M. Mansell, Dalton Trans., 2017, 46, 15157.
R. J. Newland, A. Smith, D. M. Smith, N. Fey, M. J. Hanton and S. M. Mansell, Organometallics, 2018, 37, 1062.
R. J. Newland, J. M. Lynam and S. M. Mansell, Chem. Commun., 2018, 54, 5482.
3. C-H Activation of unfunctionalised arenes and alkynes
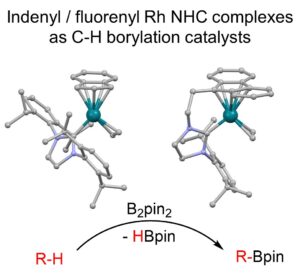
We have identified new rhodium catalysts for the C-H borylation of both arenes and alkanes. Our recent paper, judged as a Hot Paper by reviewers, describes the synthesis of indenyl and fluorenyl-tethered Rh catalysts with NHC donors, and demonstrate that we can tune the reactivity of the Rh centre through ligand substitution.
Collaborators: Jason Lynam (York)
Publications
K. J. Evans, P. A. Morton, C. Luz, C. Miller, O. Raine, J. M. Lynam and S. M. Mansell, Chem. Eur. J., 2021, https://doi.org/10.1002/chem.202102961
4. Synergy in Multimetallic Complexes: Synthesis and Catalysis
We have described recently two lithium amide/lithium phenyl reagents that deliver unique reactivity. These reagents were successful where conventional bases were not in the deprotonative ring opening synthesis of a tethered NHC from a spirocyclic compound. The unique reactivity of these synergic reagents allowed us to synthesise dilithium, disodium and dipotassium salts of a fluorenyl-tethered NHC.

Publications
K. J. Evans and S. M. Mansell, Chem.-Eur J., 2019, 25, 3766.
S. G. Rachor, P. A. Cleaves, S. D. Robertson and S. M. Mansell, J. Organomet. Chem., 2018, 857, 101.
K. J. Evans, C. L. Campbell, M. F. Haddow, C. Luz, P. A. Morton and S. M. Mansell, Eur. J. Inorg. Chem., 2019, 4894.
K. J. Evans and S. M. Mansell, Chem.-Eur J., 2020, 26, 5927.
M. Rosello-Merino and S. M. Mansell, Dalton Trans., 2016, 45, 6282.
K. J. Evans, P. A. Morton, C. Sangster and S. M. Mansell, Polyhedron, 2021, 197, 115021
5. New horizons in polymerisation
we have developed a new family of neutral Ni complexes for the formation of polyethylene. These tolerant catalysts were targeted for copolymerisations, and the use of this family of complexes in copolymerisations with polar monomers will be explored. In particular, we see great benefit in the copolymerisation of CO with ethene, generating polyketone, as we see that CO2-derived CO could be a useful way to lock up carbon that would otherwise have been released into the environment.
Collaborators: Dr Ruaraidh McIntosh (HWU), Prof. Paul Kamer (LIKAT).
 Publications
Publications
M. J. Andrews, P. Ewing, M. C. Henry, M. Reeves, P. C. J. Kamer, B. H. Muller, R. D. McIntosh and S. M. Mansell, Organometallics, 2020, 39, 1751
